Research
 I am fundamentally interested in the abundance and diversity we observe in nature, and particularly in mosquitoes. My research questions span ecology, genomics, and macroevolutionary hypothesis testing to gain insight into the abundance and diversity of disease vectors. More generally, I enjoy collaborating across a wide range of systems to understand how species and populations evolve and diverge.
I am fundamentally interested in the abundance and diversity we observe in nature, and particularly in mosquitoes. My research questions span ecology, genomics, and macroevolutionary hypothesis testing to gain insight into the abundance and diversity of disease vectors. More generally, I enjoy collaborating across a wide range of systems to understand how species and populations evolve and diverge.
As a member of the Wiegmann Lab at North Carolina State University, I employ genomic tools to understand evolution in flies. As part of a National Science Foundation-funded project to reconstruct a species-level phylogeny of mosquitoes, I am using existing genomic resources of mosquitoes to aid in sequence capture of orthologous loci across thousands of mosquito species. Ultimately, our aim with this project is to provide a comprehensive understanding of the evolutionary relatedness of mosquito species, such that we can test important hypotheses on the origin of key vector-related traits, ranging from blood host feeding to larval habitat preferences. 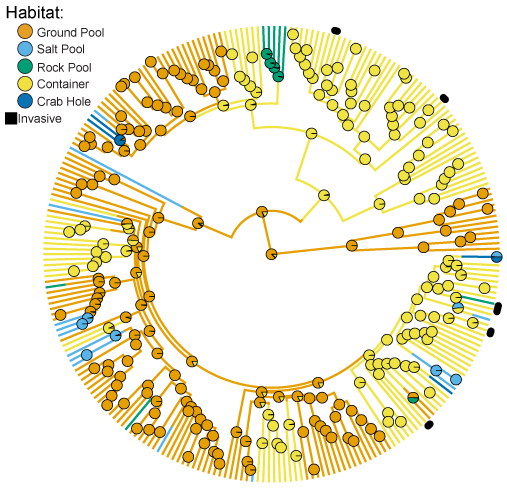 As an example of the kind of questions we are interested in, in a previous study, I demonstrated that in the genus Aedes, home to some of the worlds deadliest disease vectors, the usage of container habitats for larval stages evolved convergently across multiple lineages over tens of millions of years.1 I also demonstrated that, along with this convergence in habitat preference, there was adaptation to larval habitat on the part of a noticeable shift in morphology.
As an example of the kind of questions we are interested in, in a previous study, I demonstrated that in the genus Aedes, home to some of the worlds deadliest disease vectors, the usage of container habitats for larval stages evolved convergently across multiple lineages over tens of millions of years.1 I also demonstrated that, along with this convergence in habitat preference, there was adaptation to larval habitat on the part of a noticeable shift in morphology.
I am also interested in population-level variation that shapes contemporary distributions and behaviors of invasive and native disease vectors. For instance, the majority of mosquito species are specialized to specific types of blood hosts, with many genera ‘specializing’ on a class of animal host. Some species of disease vectors may feed predominantly on one class of bloodhosts, but occasionally, populations exhibit variability in feeding patterns that are not related to abundance of bloodhosts. This is the case with Culiseta melanura, the primary vector of Eastern Equine Encephalitis virus (EEE) and the focus of one of my projects during my postdoc at the Connecticut Agricultural Experiment Station. EEE is a rare but extremely deadly vector borne disease, which until recently, occurred in sporadic outbreaks in isolated focal locations. In the first study2 to evaluate the population structure of this important species, we found evidence for a cryptic and highly divergent lineage in some northeastern populations, as well as fine-scale structure between northern and southern populations. However, we also found strong evidence of gene flow between populations, indicating a connectivity in the northeast that was previously unknown. I discuss these results in greater detail elsewhere on my site.
 During my doctoral work with Dr. Todd Livdahl, I focused primarily on the ecology of host-pathogen associations in mosquitoes, with a particular focus on Ascogregarina, a genus of parasites found exclusively in container mosquitoes. Much of my dissertation involved characterizing their interactions with host mosquitoes. Ascogregarina develop entirely in larval phases of the mosquito and are ubiqutous with hosts, making them easy to culture in the lab and use as experimental treatments in microcosms. Ascogregarina barretti, a species of Ascogregarina that infects Aedes triseriatus, is shown at left. These parasites show striking adaptation to host - from detecting the sex of the mosquito they are infecting 3, to altering interactions between larval mosquitoes and one of their predators 4. In the future, I hope to return to the Ascogregarina system and further study their effects on mosquito communities.
During my doctoral work with Dr. Todd Livdahl, I focused primarily on the ecology of host-pathogen associations in mosquitoes, with a particular focus on Ascogregarina, a genus of parasites found exclusively in container mosquitoes. Much of my dissertation involved characterizing their interactions with host mosquitoes. Ascogregarina develop entirely in larval phases of the mosquito and are ubiqutous with hosts, making them easy to culture in the lab and use as experimental treatments in microcosms. Ascogregarina barretti, a species of Ascogregarina that infects Aedes triseriatus, is shown at left. These parasites show striking adaptation to host - from detecting the sex of the mosquito they are infecting 3, to altering interactions between larval mosquitoes and one of their predators 4. In the future, I hope to return to the Ascogregarina system and further study their effects on mosquito communities.
-
Soghigian et al. 2017. From ground pools to treeholes: convergent evolution of habitat and phenotype in Aedes mosquitoes, BMC Evolutionary Biology ↩
-
Soghigian et al. 2018 Population genomics of Culiseta melanura, the principal vector of Eastern equine encephalitis virus in the United States. PLoS Neglected Tropical Diseases ↩
-
Soghigian, J. and Livdahl, T.P. 2017. Differential response to mosquito host sex and parasite dosage suggest mixed dispersal strategies in the parasite Ascogregarina taiwanensis. PloS ONE ↩
-
Soghigian et al. 2017. A parasite’s modification of host behavior reduces predation on its host. Ecology and Evolution, 7:1453–1461. ↩
